Highlights
350+
Healthcare IT Specialists
100+
HIT Products Developed
50+
Successful HIT Certifications
Medical Device Software Development Services is the process of design, development, and testing of software applications that are responsible for the seamless operation of medical devices. At Nalashaa Solutions, we deliver compliant, secure, high-performance software adhering to global regulatory standards. Our solutions enable healthcare providers to streamline diagnostics, improve decision-making, and ensure the reliability of medical devices in various healthcare settings.
Build your Legacy
- Our team of consultants/business analysts drive requirements management
- Solution specialists/architects drive the solution design and development
- Employ Lean SDLC methodologies and usage of accelerators to reduce time-to-market
- Prioritized Product/Release backlog to develop functional components, MVP, and overall solution
- Acceptance criteria, Definition of Done and Go-Live as per the Release plan

- In-depth knowledge of regulatory and product compliance needs, e.g., MU, PPACA, ICD-10, FDA
- Consulting team, comprising SMEs, keeps track of emerging regulatory trends
- Gap analysis and recommendations for any updates required to the product/solution
- Extensive knowledge of healthcare data standards – FHIR, DIRECT, CCDA
- Thorough process fine-tuning and compliance
- Solid skills in new product/solution development and sustenance
- Experienced in a variety of technologies and flexible delivery models
- Professional services for remote monitoring, sustenance, enhancements, and quick fixes
- Strong engineering team for implementations, customizations, integrations, and support

- Expert SDETs on team to ensure optimal test coverage through V&V
- Robust push for QA automation wherever feasible and ROI is evident
- Performance testing using tools such as LoadRunner, WebLOAD, LoadNinja, JMeter
- Security and vulnerability conformance to OWASP/OSSTMM/NIST/PTES/ISSAF standards
- Regulatory testing to assess process workflow, technology, and data requirements for compliance
- Interoperability testing to ensure secure information exchange between integrated applications/devices

- Extensive experience in application/device/workflow/data integration
- Experienced team of integration/interoperability engineers
- Thorough knowledge of standards such as FHIR/HL7, CCDA, QRDA, IHE, DICOM, X12 EDI
- Experience working with integration engines like Mirth, Cloverleaf, Rhapsody, Iguana

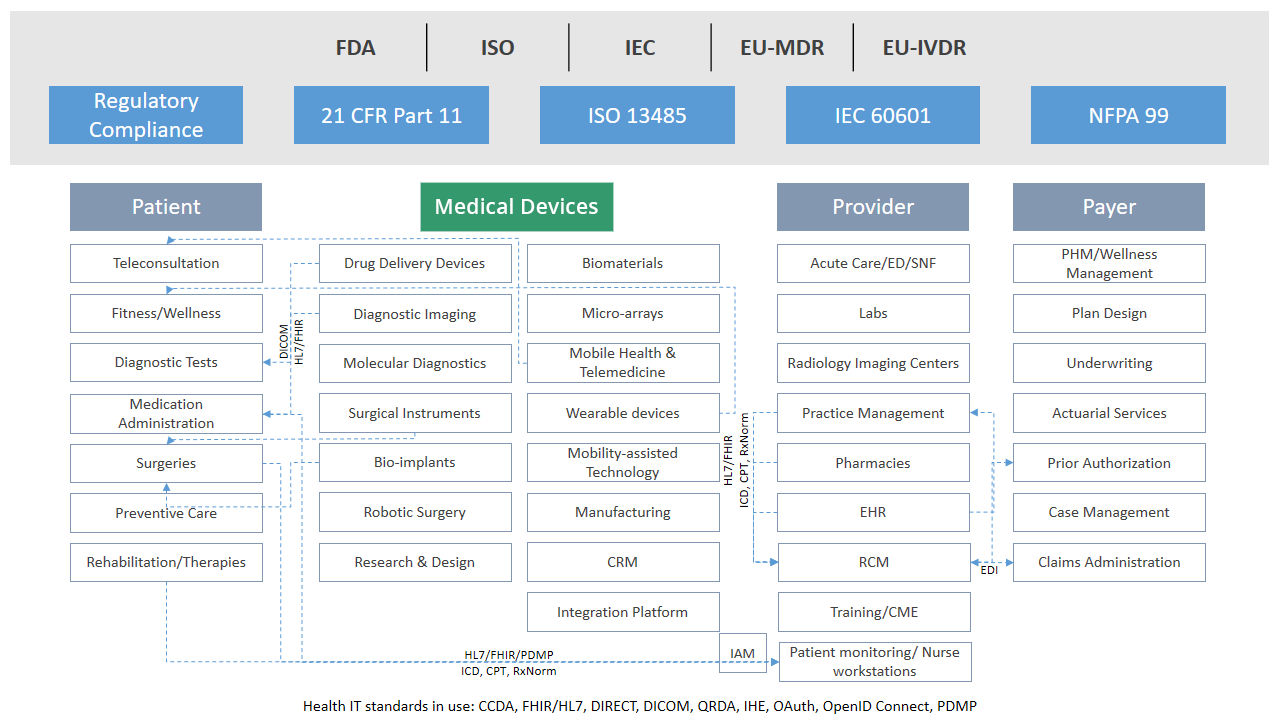
The Devices Ecosystem
Let’s Collaborate to Create
Let’s Collaborate to
Create
Innovate
With seasoned expertise in medical device engineering services and regulatory compliances, we can arm you with the right solutions.
Connect with our experts to chalk out a roadmap for the same.
Worth Exploring
A glimpse of our industry expertise
