To further the growth of your organization, your strategy may be to develop a new product, develop software as a medical device (SaMD), achieve device miniaturization, to develop embedded software for a medical device, and/or to apply data analytics. Stay on top of your game with Nalashaa.
As an Agile partner, Nalashaa can help you in realizing your strategy through its proficiencies in MedTech engineering, leveraging our Healthcare IT expertise.
What Nalashaa can do as your Agile Partner
Going Digital
- Devices integration & interoperability in an IoMT ecosystem
- Leverage HL7/FHIR expertise
- Mobile and Cloud enablement
- Device cybersecurity
Customer Experience Engineering
- Attention to details of user requirements and user feedback
- Responsive (Web) design
- User-Centered Design (UCD)
- Effective microinteractions
SaMD Development
- Collect massive amounts of accurate data
- Detect and diagnose a health condition
- Aids in improving health outcomes
- Aids in innovation of tools
QA for Medical Devices
- Medical Devices and Simulation Testing
- Documentation of entire QA process
- Validation Master Plan in accordance with cGMP
- QA Automation
Data Analytics
- Before – Market analysis, demand forecast, competitive landscape, feature set study
- During – Risk management, customizations, supply chain management
- After – Usage analysis, future product roadmap determination, pre-empting product recalls
Our Approach
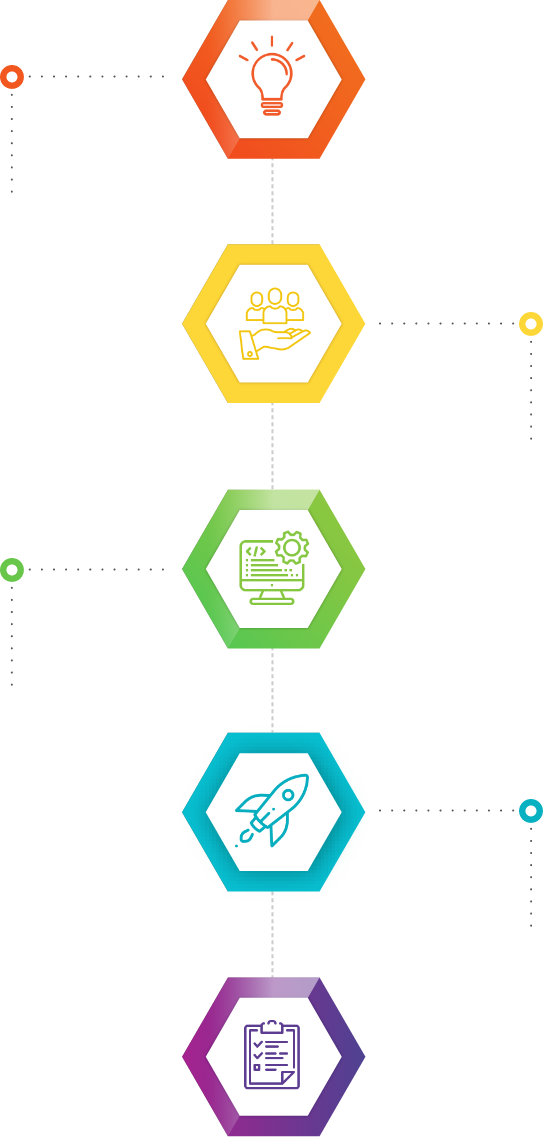
Initiation
Ideate | Identify product differentiators | Decide the market | Determine the demand | Use a QMS | Estimate the risk-to-reward ratio | Analyze competitors | Analyze legal/IP documents
Formulation
Form core team | Create project plan | Develop the concept/prototype | Initiate DHF | Initiate regulatory strategy | Initiate risk management | Plan for clinical trials, if required
Design & Development
Design test Strategy, scenarios, cases | Manage risks | Maintain DHF | Regulatory submission | Start IQ/OQ/PQ validation | Prepare clinical validation
Product Launch preparation
Brand the product | Plan product launch | Complete DHF | Review and update risk management | Create mechanism to gather user feedback | Get regulatory clearance | Validate the final process
Product Launch & Post Launch Assessment
End user training and support | Capture end user feedback | Post market observation and troubleshooting, if required | Make process improvements | Update design documents | Conduct quality audits
Our Approach
Initiation
Ideate | Identify product differentiators | Decide the market | Determine the demand | Use a QMS | Estimate the risk-to-reward ratio | Analyze competitors | Analyze legal/IP documents
Formulation
Form core team | Create project plan | Develop the concept/prototype | Initiate DHF | Initiate regulatory strategy | Initiate risk management | Plan for clinical trials, if required
Design & Development
Design test Strategy, scenarios, cases | Manage risks | Maintain DHF | Regulatory submission | Start IQ/OQ/PQ validation | Prepare clinical validation
Product Launch preparation
Brand the product | Plan product launch | Complete DHF | Review and update risk management | Create mechanism to gather user feedback | Get regulatory clearance | Validate the final process
Product Launch & Post Launch Assessment
End user training and support | Capture end user feedback | Post market observation and troubleshooting, if required | Make process improvements | Update design documents | Conduct quality audits
Why Our Clients Love Us?
Focus on Medical Devices
We specialize in healthcare IT solutions & services
Interoperability Experts
HL7 and FHIR expertise for flawless device connectivity
QA
for MedTech
Experience in verification & validation
Accountability and Transparency
Responsible teams providing you with unhindered visibility
